Slezak T, Bailey LJ, Jaskolowski M, Nahotko DA, Filippova EV, Davydova EK, Kossiakoff AA
Protein Sci. 2019 Oct;
PMID: 31622515
Abstract
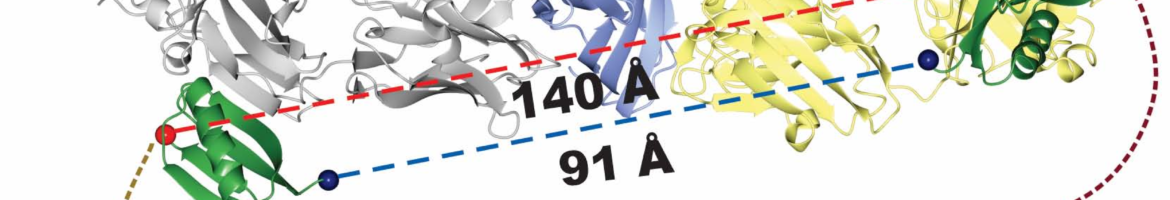
Engineered recombinant antibody-based reagents are rapidly supplanting traditionally derived antibodies in many cell biological applications. A particularly powerful aspect of these engineered reagents is that other modules having myriad functions can be attached to them either chemically or through molecular fusions. However, these processes can be cumbersome and do not lend themselves to high throughput applications. Consequently, we have endeavored to develop a platform that can introduce multiple functionalities into a class of Fab-based affinity reagents in a “plug and play” fashion. This platform exploits the ultra-tight binding interaction between affinity matured variants of a Fab scaffold (Fab ) and a domain of an immunoglobulin binding protein, protein G (GA1). GA1 is easily genetically manipulatable facilitating the ability to link these modules together like beads on a string with adjustable spacing to produce multivalent and bi-specific entities. GA1 can also be fused to other proteins or be chemically modified to engage other types of functional components. To demonstrate the utility for the Fab-GA1 platform, we applied it to a detection proximity assay based on the β-lactamase (BL) split enzyme system. We also show the bi-specific capabilities of the module by using it in context of a Bi-specific T-cell engager, which is a therapeutic assemblage that induces cell killing by crosslinking T-cells to cancer cells. We show that GA1-Fab modules are easily engineered into potent cell killing BiTE-like assemblages and have the advantage of interchanging Fabs directed against different cell surface cancer related targets in a plug and play fashion. This article is protected by copyright. All rights reserved.